Gout results from the deposition of monosodium urate (MSU) crystals in joints and tissues, secondary to hyperuricemia. Hyperuricemia occurs when serum urate levels exceed the physiological saturation point (6.8 mg/dL), leading to urate crystal formation. The pathogenesis involves:
- Urate Overproduction: Due to increased purine metabolism or reduced degradation. Conditions like myeloproliferative disorders, psoriasis, or increased dietary purine intake contribute.
- Decreased Renal Excretion: The kidneys excrete two-thirds of uric acid, with the rest eliminated via the gastrointestinal tract. Factors impairing excretion include chronic kidney disease, diuretics (thiazides, loop diuretics), cyclosporine, and lead nephropathy.
- Enzyme Defects: Mutations in PRPP synthetase (overactivity) or HGPRT deficiency (as seen in Lesch-Nyhan syndrome) enhance purine production or reduce recycling, increasing urate levels.
Inflammatory response begins when MSU crystals activate NLRP3 inflammasomes in resident macrophages, leading to caspase-1 activation and IL-1β production. Subsequent cytokine cascade recruits neutrophils, promoting a robust inflammatory response.
Clinical Presentation
- Acute Gout Attack
- Typically presents as a sudden onset of severe joint pain, erythema, swelling, and warmth, often at night.
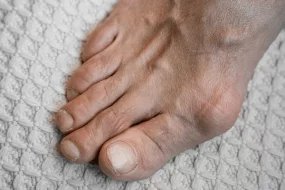
- Podagra (1st MTP joint) is the classic presentation, but other commonly affected joints include the ankles, knees, wrists, and elbows.
- Differential diagnoses include septic arthritis, cellulitis, pseudogout, and other forms of crystal arthropathies (e.g., calcium pyrophosphate deposition disease).

- Intercritical Gout
- Asymptomatic periods between attacks. Urate deposition continues in tissues even during this phase.
- Without ULT, attacks become more frequent and involve more joints over time.
- Chronic Tophaceous Gout
- Chronic phase with persistent low-grade inflammation and formation of tophi in joints, bursae, tendons, or cartilage.
- Tophi are palpable deposits of urate that may ulcerate and discharge chalky material.
- Chronic tophaceous gout may lead to deformity, joint destruction, and reduced mobility.
Diagnosis
- Clinical Examination
- Red, swollen, and painful joints are typical.
- Tophi may appear as nodular, subcutaneous lesions.
- Synovial Fluid Analysis
- Needle-shaped, negatively birefringent MSU crystals are diagnostic.
- Synovial fluid may appear cloudy with elevated leukocyte count (5,000 to 80,000 cells/μL, predominantly neutrophils).
- Laboratory Tests
- Serum Uric Acid: Hyperuricemia supports the diagnosis but is not definitive since levels may be normal during an acute attack. Aim for levels <6 mg/dL or <5 mg/dL in tophaceous gout for control.
- Renal Function Tests: Assess for chronic kidney disease or renal insufficiency.
- Complete Blood Count and ESR/CRP: Elevated during acute inflammation.
- Imaging
- X-ray: Advanced gout shows punched-out erosions with sclerotic margins, often overhanging edges (rat-bite appearance).
- Dual-Energy CT: Allows non-invasive detection of MSU crystals.
- Ultrasound: Double-contour sign (urate deposition on the cartilage) and tophi detection.
Management
1. Acute Gout Attack Management
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
- First-line agents. Common choices include:
- Indomethacin: 50 mg TID for 2-3 days, then tapering to 25 mg BID for another 5-7 days.
- Naproxen: 750 mg initially, followed by 250 mg every 8 hours.
- Ibuprofen: 800 mg TID, dose adjustment based on response and patient tolerance.
- Avoid in patients with peptic ulcer disease or renal insufficiency.
- First-line agents. Common choices include:
- Colchicine
- Effective within the first 36 hours of symptom onset.
- Dosage: 1.2 mg initially, followed by 0.6 mg one hour later. Maintenance dose of 0.6 mg once or twice daily for prophylaxis.
- Caution in renal or hepatic impairment; dose adjustment required.
- Corticosteroids
- Systemic corticosteroids for polyarticular gout or when NSAIDs/colchicine are contraindicated.
- Prednisone: 30-60 mg daily for 5-10 days, then taper.
- Intra-articular injection: Triamcinolone acetonide 40 mg for larger joints or 10-20 mg for smaller joints.
- Systemic corticosteroids for polyarticular gout or when NSAIDs/colchicine are contraindicated.
- Interleukin-1 Inhibitors (Refractory Cases)
- Anakinra: 100 mg subcutaneously daily for 3 days.
- Canakinumab: 150 mg subcutaneously (approved in some countries for resistant cases).
2. Chronic Urate-Lowering Therapy (ULT)
Indications: Recurrent gout attacks (≥2/year), tophi, joint erosions, chronic kidney disease (CKD stage ≥2), or urate nephropathy.
- Xanthine Oxidase Inhibitors
- Allopurinol: Starting dose 100 mg daily, titrated up every 2-4 weeks until serum uric acid <6 mg/dL.
- Maximum dose can reach 800 mg/day. Monitor for hypersensitivity, especially in patients with HLA-B*5801 (more common in Han Chinese, Thai, and Korean populations).
- Febuxostat: Alternative to allopurinol, starting at 40 mg daily, increasing to 80 mg if serum urate remains >6 mg/dL.
- Contraindicated in patients with a history of cardiovascular disease (FDA warning).
- Allopurinol: Starting dose 100 mg daily, titrated up every 2-4 weeks until serum uric acid <6 mg/dL.
- Uricosuric Agents
- Probenecid: Enhances renal excretion of uric acid. Dose: 250 mg BID, increasing by 500 mg every 4 weeks, up to a maximum of 2 g/day.
- Avoid in patients with a history of uric acid nephrolithiasis or overproduction of urate.
- Probenecid: Enhances renal excretion of uric acid. Dose: 250 mg BID, increasing by 500 mg every 4 weeks, up to a maximum of 2 g/day.
- Pegloticase
- Recombinant uricase enzyme for treatment-refractory gout.
- Dosage: 8 mg IV every 2 weeks. Monitor for anaphylaxis and infusion reactions.
3. Lifestyle and Dietary Management
- Dietary Recommendations
- Limit purine-rich foods (red meat, seafood), alcohol (especially beer), and fructose-rich beverages.
- Encourage low-fat dairy products, cherries, and coffee intake, which may lower uric acid levels.
- Hydration: Maintain adequate fluid intake (2-3 liters/day) to prevent urate nephrolithiasis.
- Weight Management
- Gradual weight loss can help lower serum uric acid levels and reduce the risk of gout attacks.
4. Monitoring
- Regularly assess serum uric acid levels every 3-6 months, aiming for <6 mg/dL.
- Monitor renal function and signs of ULT-related adverse effects.
- Evaluate for changes in the size and number of tophi with ULT.
Complications
- Chronic Kidney Disease (CKD) and Urolithiasis: Associated with longstanding hyperuricemia.
- Cardiovascular Disease: Hyperuricemia is an independent risk factor for hypertension and other cardiovascular events.
- Joint Deformities: Result from chronic inflammation and tophi deposition.